*Use of Alteogen-based Technology / **Possession of business rights of Alteogen production goods
Tacrolimus (Development of Ophthalmic Agent)
Autoimmune Disease
- Known autoimmune diseases → 80 total kinds
-
A typical autoimmune disease
- A typical autoimmune disease
- Rheumatoid arthritis
- Fibromyalgia
- Psoriasis
- Alphosis
- Uveitis
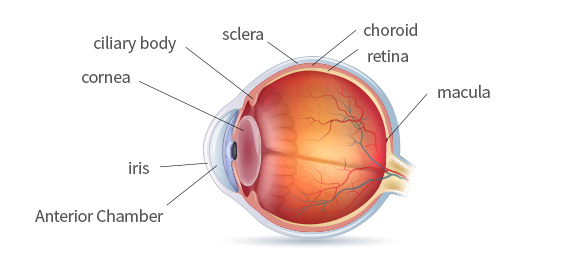
Structure of the Eye
Typical Treatment of Autoimmune Uveitis
-
Area of Occurrence : iris, Ciliary Body, Choroid
※ This area is rich in blood vessels and connective tissue, and it is easy to develop -
Treatment: Use of local or systemic steroids
→ However, when used in high doses, it can cause serious side effects
There is a need to develop a treatment
for eye diseases that will replace steroids
for eye diseases that will replace steroids
Treatment of Dry Eye (Allergic Conjunctivitis)
- Treatment with Light Symptoms: Antihistamines and steroids
- Treatment when Severe and Acute: Use of Cyclosporine-A (CCP) ophthalmic (Restasisⓡ) as an immunosuppressant
- CCP is prepared as an emulsion at a low concentration of 20 ug/ml. Common problems with stability include condensation, precipitation, creaming, crystallization, adhesion, etc.
There is a need to develop a treatment
for eye diseases that will replace steroids
for eye diseases that will replace steroids
Technical Overview of Our New and Enhanced Tacrolimus Product (WO2013022238)
Background and Objectives for Developing Our New and Enhanced Substance
- To replace steroid treatments with large side effects
- To develop a stable, competitive model with a low unit cost of production (Production of emulsions require expensive equipment)
- Final goal: To develop an ointment (to maximize stability), a cream (with water soluble product), and an ophthalmic preparation
The Advantages of Our Technology
-
Characteristics of TAC Containing Tacrolimus
- Most TAC are non-polar and do not dissolve well in water (Solubility in water 4-12 ug/ml).
- Due to the low hydrophilicity, it is difficult to develop other modes of administration, such as injectable, and the pharmacokinetic bioavailability is very low.
- TACs, such as tacrolimus, are prone to degradation when dissolved in organic solvents and rapidly administered as an injectable. Due to low solubility, such administrations can result in precipitation and crystallization of the compound.
- Glycosylated tacrolimus, synthesized by our patented technology, is a more chemically stable compound with a higher water solubility (~1000x that of TAC). Glycosylated tacrolimus and its derivatives have been developed to identify candidate substances for our new, improved drug product.
