Vancomycin Hydrochloride (Antibiotic)
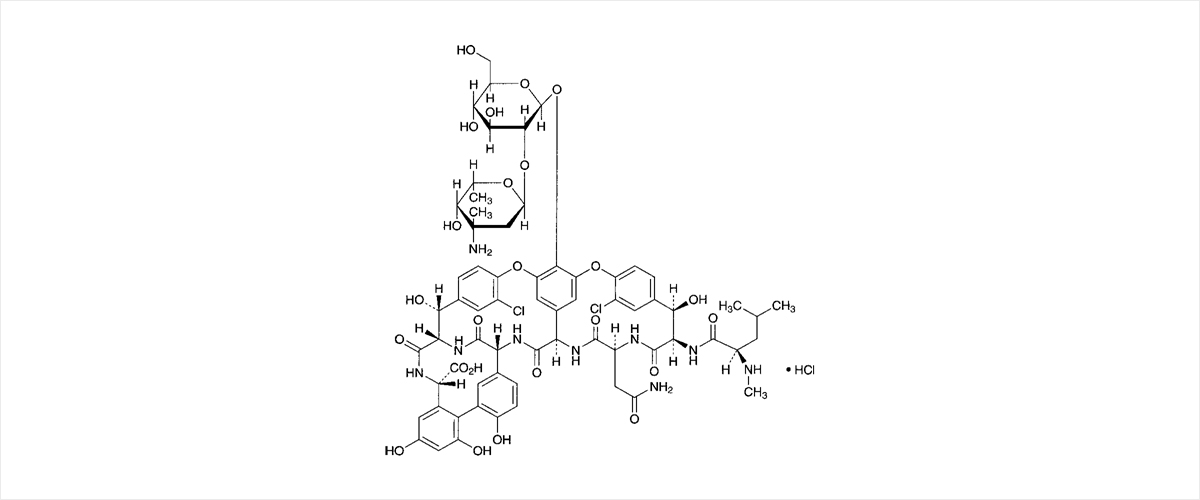
C66H75CI2N9O24·HCI
- Vancomycin is a glycopeptide antibiotic with a cyclic heptapeptide core aglycone, which is a difficult structure for chemical synthesis. It has bactericidal activity that inhibits cell wall synthesis and is used to treat infections due to Gram-positive bacteria. It is used to treat infections due to bacteria that are resistant to antibiotics, like penicillin or cephalosporin.
- Vancomycin is produced mainly by industrial fermentation. It is typically administered intravenously and used to treat various infections, such as sepsis, skin infections, urinary tract infections, and intestinal infections. It is also administered as oral or ophthalmic agents for treatment of intestinal and eye disease infections.
1. Korea Patent
| NO. | Patent | Register | Register No. |
|---|---|---|---|
| 1 | Process for Purifying Vancomycin Wet Body | 2011-12-26 | 10-1101663-0000 |
| 2 | anscription regulating sigma factor SigA-vm and producing method for glycopeptide compounds by overexpressing the sigma factor |
2016-02-01 | 10-1592269-0000 |
| 2 | Method for increasing the productivity of glycopeptides compounds | 2017-06-13 | 10-1748678-0000 |
CERTIFICATE OF ANALYSIS
Product Name : Vancomycin Hydrochloride (Vancomycin)| Test | Specification | |
|---|---|---|
| Appearance | A white or almost white powder | |
| Solubility | Freely soluble in water, very slightly soluble in ethanol 96% | |
| Identification | IR | The IR spectrum of sample should be concordant with that of standard |
| pH | 2.5 – 4.5 | |
| Water | NMT 5.0% | |
| Assay | NLT 900ug/mg (anhydrous) | |
| Vancomycin B purity | Vancomycin B | NLT 85% |
| Main Impurity | NMT 5.0% | |
| Monodechlorovancomycin | NMT 4.7% | |
| Residual organic solvent - ethanol | NMT 5000 ppm | |
| Sterility | Sterile | |
| Bacterial Endotoxin | NMT 0.33 unit/mg | |
| This is USP version. Specification will be changed for IP/JP/Ph.Eur | ||
