Teicoplanin (Antibiotic)
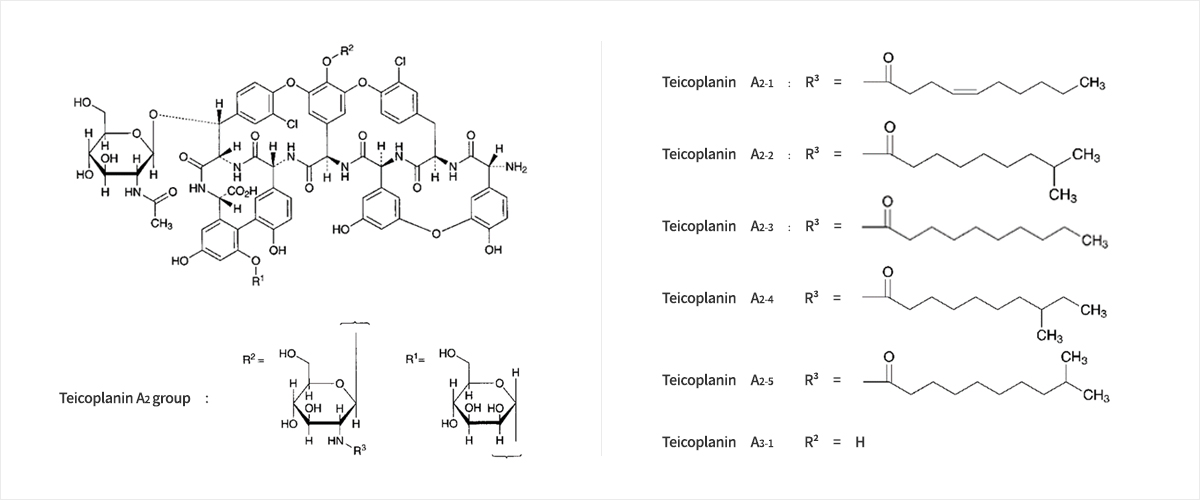
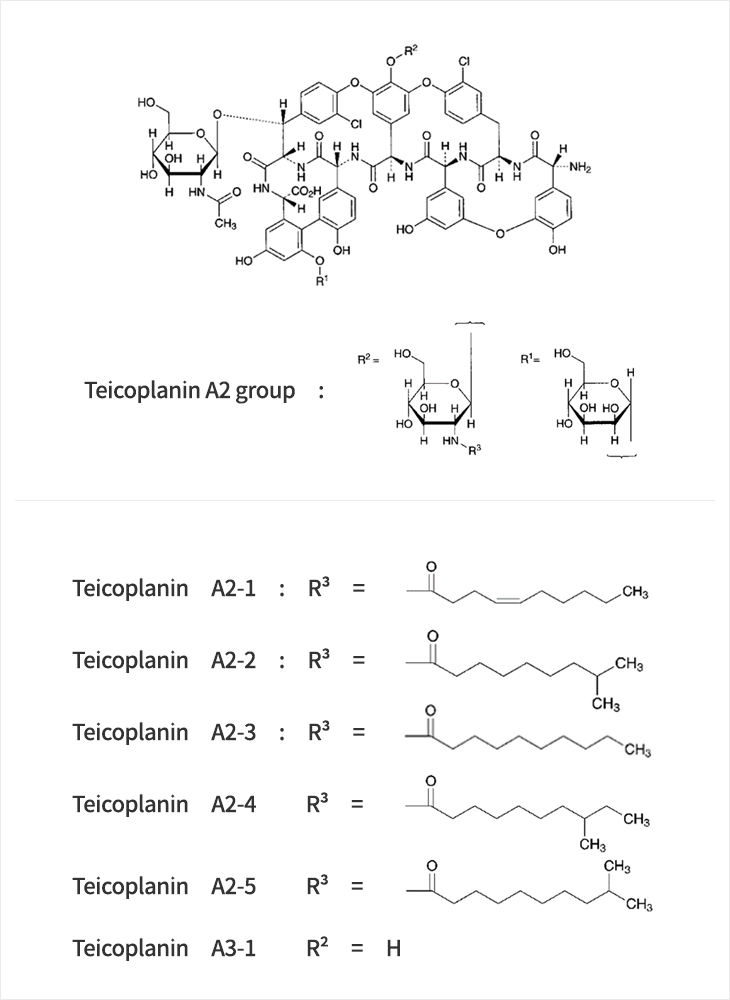
C72H68CI2N8O28
- Teicoplanin is an antibiotic prescribed as a last resort for patients infected with superbacteria, such as MRSA (methicillin-resistant Staphylococcus aureus) and VRE (vancomycin-resistant enterococci). Teicoplanin is a mixture of several compounds, five major in the teicoplanin A2 group and four minor in the teicoplanin Rs group, which all have a glycopeptide common core termed teicoplanin A3-1.
- Glycopeptide antibiotics, like teicoplanin, are highly valued antibiotics with increasing demand. They are particularly effective in treating diseases caused by the infection of Gram-positive bacteria. They can be administered by injection into muscle once a day due to a long half-life.
- High-quality teicoplanin commercial production is possible due to process validation of freeze-dried raw materials (aseptic products) produced from the development of a high production mutant strain, independent fermentation, and purification processes at the Ceres F&D Jecheon Plant.
1. Korea Patent
| NO. | Patent | Register | Register No. |
|---|---|---|---|
| 1 | Recovery method for teicoplanin | 2017-11-22 | 10-1802191-0000 |
| 2 | Purification method of teicoplanin | 2018-03-28 | 10-1844833-0000 |
CERTIFICATE OF ANALYSIS
Product Name : TeicoplaninEP (Sterile)| Test | Specification | |
|---|---|---|
| Description | Appearance | Yellowish, amorphous powder |
| Solubility | Freely soluble in water, sparingly soluable in dimethylformamide, practically insoluable in ethanol (96) | |
| Identification | IR | Concordant with reference Standard |
| HPLC | Concordant with reference Standard | |
| Appearance of solution | The solution is clear and not more intensely colored than reference solution BY3 or B4 | |
| pH (0.5 g per 10mL) | Between 6.5 ~ 7.5 | |
| Composition and related substances (by HPLC) | Teicoplanin A2 group | Not less than 80.0% |
| Teicoplanin A2-1 group | Not more than 20.0% | |
| Teicoplanin A2-2 | 35.0 to 55.0% | |
| Teicoplanin A2-3 group | Not more than 20.0% | |
| Teicoplanin A2-4 | Not more than 20.0% | |
| Teicoplanin A2-5 group | Not more than 20.0% | |
| Teicoplanin A3 group | Not more than 15.0% | |
| Total impurities other than mesityl oxide | 1.25 to 5.0% | |
| Chlorides (Calculated on NaCl and anhydrous basis | Not more than 5.0% | |
| Heavy metals | Not more than 0.002 % | |
| Impurity A (Mesityl Oxide) | Not more than 0.2% | |
| Water [%w/w by KF] | Not more than 15.0 % | |
| Bacterial Endotoxins | Not more than 0.31IU/mg | |
| This is EP version. Specification will be changed for USP/IP/JP/Ph.Eur | ||
